S Designations
—See supports for gas chromatography in the
Chromatographic Reagents section under
Chromatography  621
621
.
Saccharose
—Use Sucrose (NF monograph).
Safranin O
—Dark red powder consisting of a mixture of 3,7-diamino-2,8-dimethyl-5-phenylphenazinium chloride, C
20H
19ClN
4—350.85, and 3,7-diamino-2,8-dimethyl-5-
o-tolylphenazinium chloride, C
21H
21ClN
4—364.88—Sparingly soluble in 70 percent alcohol yielding a clear red solution with a yellowish-red fluorescence.
Identification—
A:

To 10 mL of a 0.5% w/v solution add 5 mL of hydrochloric acid: a bluish violet solution is produced.
B:

To 10 mL of a 0.5% w/v solution add 5 mL of sodium hydroxide solution (1 in 5): a brownish-red precipitate is produced.
C:

To 100 mg add 5 mL of sulfuric acid: a green solution is produced, which, on dilution, changes to blue and finally to red.
Absorption characteristics—
Dissolve 50 mg in 250 mL of 50 percent alcohol. Dilute 3 mL of this solution with 50 percent alcohol to 200 mL. Determine the absorbance, in a 1-cm cell, with a suitable spectrophotometer. The absorbance maximum is in the range of 530 to 533 nm; the ratio (
P 
15)/(
P + 15) is between 1.10 and 1.32, in which
P is the wavelength of maximum absorbance.
[NOTE—A suitable grade is available as catalog number 10,214-8 from Sigma-Aldrich,
www.sigma-aldrich.com.
]
Salicylaldehyde,

2-HOC
6H
4CHO—
122.12—Clear, colorless to yellowish-green liquid. Specific gravity: about 1.17. Slightly soluble in water; soluble in alcohol and in ether. May contain a stabilizer.
Assay—
When examined by gas-liquid chromatography, using suitable apparatus and conditions, it shows a purity of not less than 98%.
Salicylaldazine,

C
14H
12N
2O
2—
240.26—Use a suitable grade or prepare as follows. Dissolve 300 mg of hydrazine sulfate in 5 mL of water, add 1 mL of glacial acetic acid and 2 mL of a freshly prepared 1 in 5 solution of salicylaldehyde in isopropyl alcohol, mix, and allow to stand until a yellow precipitate is formed. Extract the mixture with two 15-mL portions of methylene chloride. Combine the methylene chloride extracts, and dry over anhydrous sodium sulfate. Decant the methylene chloride solution, and evaporate it to dryness. Recrystallize the residue of salicylaldazine from a mixture of warm toluene and methanol (60:40) with cooling. Filter, and dry the crystals in vacuum.
Melting range  741
741 :
:

between 213

and 219

, but the range between beginning and end of melting does not exceed 1

.
Chromatography—
Proceed as directed in Limit of hydrazine under Povidone: the chromatogram shows only one spot.
Sand, Standard 20- to 30-Mesh
—Silica sand, composed almost entirely of naturally rounded grains of nearly pure quartz. Predominantly graded to pass an 850-µm (No. 20) sieve (85 to 100 percentage passing) and be retained on a 600-µm (No. 30) sieve (0 to 5 percentage passing).
[NOTE—A suitable grade is available as Ottawa Standard Sand from Thomas Scientific, 99 High Hill Road at I-295, P.O. Box 99, Swedesboro, NJ 08085-0099.]
Sand, Washed
—It may be prepared as follows. Digest clean, hard sand at room temperature with a mixture of 1 part of hydrochloric acid and 2 parts of water (about 13% of HCl) for several days, or at an elevated temperature for several hours. Collect the sand on a filter, wash with water until the washings are neutral and show only a slight reaction for chloride, and finally dry. Washed sand meets the following tests.
Substances soluble in hydrochloric acid—
Digest 10 g with a mixture of 10 mL of hydrochloric acid and 40 mL of water on a steam bath for 4 hours, replacing from time to time the water lost by evaporation. Filter, and to 25 mL of the filtrate add 5 drops of sulfuric acid, evaporate, and ignite to constant weight: the residue weighs not more than 8 mg (0.16%).
Chloride (Reagent test)—
Shake 1 g with 20 mL of water for 5 minutes, filter, and add to the filtrate 1 mL of nitric acid and 1 mL of
silver nitrate TS: any turbidity produced corresponds to not more than 0.03 mg of Cl (0.003%).
Sawdust, Purified
—It may be prepared as follows. Extract sawdust in a percolator, first with sodium hydroxide solution (1 in 100), and then with dilute hydrochloric acid (1 in 100) until the acid percolate gives no test for alkaloid with mercuric-potassium iodide TS or with
iodine TS. Then wash with water until free from acid and soluble salts, and dry. Purified sawdust meets the following test.
Alkaloids—
To 5 g of purified sawdust contained in a flask add 50 mL of a mixture of 2 volumes of ether and 1 volume of chloroform and 10 mL of
ammonia TS, and shake frequently for 2 hours. Decant 20 mL of the clear, ether-chloroform liquid, and evaporate to dryness. Dissolve the residue in 2 mL of dilute hydrochloric acid (1 in 12), and divide into two portions. To 1 portion add mercuric-potassium iodide TS, and to the other add iodine TS: no turbidity is produced in either portion.
Scandium Oxide,

Sc
2O
3—
137.91


[
12060-01-1]—Fine, white powder.
Selenious Acid
(
Selenous Acid),

H
2SeO
3—
128.97—Colorless or white crystals, efflorescent in dry air and hygroscopic in moist air. Soluble in water and in alcohol.
Assay—
Accurately weigh about 100 mg, transfer to a glass-stoppered flask, and dissolve in 50 mL of water. Add 10 mL of potassium iodide solution (3 in 10) and 5 mL of hydrochloric acid, mix, insert the stopper in the flask, and allow to stand for 10 minutes. Dilute with 50 mL of water, add 3 mL of starch TS, and titrate with 0.1 N sodium thiosulfate VS until the color is no longer diminished, then titrate with 0.1 N iodine VS to a blue color. Subtract the volume of 0.1 N iodine solution from the volume of 0.1 N sodium thiosulfate to give the volume of 0.1 N thiosulfate equivalent to selenious acid. Each mL of 0.1 N sodium thiosulfate is equivalent to 3.225 mg of H2SeO3: not less than 93% is found.
Insoluble matter—
Dissolve 1 g in 5 mL of water: the solution is clear and complete.
Residue on ignition (Reagent test):

not more than 1.0 mg (0.01%), from 10 g.
Selenate and sulfate—
Dissolve 500 mg in 10 mL of water, and add 0.1 mL of hydrochloric acid and 1 mL of
barium chloride TS: no turbidity or precipitate is formed within 10 minutes.
Selenium,

Se—
At. Wt. 78.96—Dark-red amorphous, or bluish-black, crystalline powder. Insoluble in water; soluble in solutions of sodium and potassium hydroxides or sulfides.
Residue on ignition (Reagent test)—
One g yields not more than 2 mg (0.2%).
Heavy metals—
To the
Residue on ignition add 3 mL of hydrochloric acid and 2 mL of nitric acid, evaporate on a steam bath to dryness, take up the residue in a mixture of 2 mL of diluted hydrochloric acid and 50 mL of hot water, cool, filter, and wash the filter with sufficient water to make 100 mL of filtrate (
Test Solution). To a 30-mL aliquot of the
Test Solution add 10 mL of water and 10 mL of
hydrogen sulfide TS: the color produced is not darker than that of a
Control Solution prepared from 3 mL of
Standard Lead Solution (see
Heavy Metals  231
231
; 0.03 mg of Pb), 0.2 mL of 1 N hydrochloric acid, 37 mL of water, and 10 mL of
hydrogen sulfide TS (0.01%).
Iron  241
241 —
—
To 20 mL of the
Test Solution prepared in the test for
Heavy metals add 2 mL of hydrochloric acid, and dilute with water to 47 mL: the solution shows not more than 0.01 mg of Fe (0.005%).
Nitrogen—
STANDARD NITROGEN SOLUTION—
Dissolve 382 mg of ammonium chloride in water to make 1 L. Each mL of this solution contains the equivalent of 0.1 mg of nitrogen (N).
PROCEDURE—
Heat 1.0 g with 10 mL of sulfuric acid in a Kjeldahl flask until the test specimen is dissolved and the volume of acid is reduced to about 5 mL. Cool, cautiously dilute with 100 mL of water, render strongly alkaline with sodium hydroxide solution (3 in 10), and distill about 75 mL of the solution into 5 mL of water containing 2 drops of 1 N hydrochloric acid. Dilute the distillate with water to 250 mL. To a 50-mL aliquot of the solution add 1 mL of sodium hydroxide solution (1 in 10) and 2 mL of mercuric-potassium iodide TS: the color produced is not darker than that produced by 0.1 mL of Standard nitrogen solution (0.01 mg of N) treated in the same manner as the test specimen (0.005%).
Sulfur—
To 1.0 g in a beaker add, successively, 5 mL of nitric acid, then 10 mL of hydrochloric acid, and evaporate on a steam bath to dryness. Add 10 mL of hydrochloric acid, and slowly evaporate again to dryness. Take up the residue in 30 mL of dilute hydrochloric acid (1 in 30), filter, and wash the filter with water to make about 100 mL of the filtrate. Heat the filtrate to boiling, and add slowly, with stirring, 5 mL of
barium chloride TS. Digest on the steam bath for 4 hours. Filter on a fine-porosity filter paper, wash the precipitate until it is free from chloride, ignite, and weigh. The weight of the barium sulfate residue, multiplied by 0.1374, represents sulfur (S). Not more than 0.5 mg of S is found (0.05%).
Selenomethionine,

C
5H
11NO
2Se—
196.11—
[Caution—Handle with care, as this reagent is highly toxic.
]
Assay—
Weigh accurately about 750 mg, dissolve in 100 mL of methanol, add crystal violet TS, and titrate with 0.1 N perchloric acid to a blue-green endpoint. Each mL of 0.1 N perchloric acid is equivalent to 19.61 mg of C5H11NO2Se: between 97.0% and 103.0%, calculated on the as-is basis, is found.
Nitrogen content  461
461 —
—
Determine by the Kjeldahl method: between 6.8% and 7.4%, calculated on the as-is basis, is found.
Silica, Chromatographic, Silanized, Flux-Calcined, Acid-washed
—Use a suitable grade.
[NOTE—Suitable grades are available commercially as “Aeropak 30,” “Diatoport S,” and “Gas-Chrom Z.”]
Silica Gel
—An amorphous, partly hydrated SiO
2 occurring in glassy granules of varying size. When used as a desiccant, it frequently is coated with a substance that changes color when the capacity to absorb water is exhausted. Such colored products may be regenerated (i.e., may regain their capacity to absorb water) by being heated at 110

until the gel assumes the original color.
[NOTE—The following procedures and limits are designed only for use in testing the desiccant grade of silica gel.
]
Loss on ignition—
Ignite 2 g, accurately weighed, at 950 ± 50

to constant weight: it loses not more than 6.0% of its weight.
Water absorption—
Place about 10 g in a tared weighing bottle, and weigh. Then place the bottle, with cover removed, for 24 hours in a closed container in which the atmosphere is maintained at 80% relative humidity by being in equilibrium with sulfuric acid having a specific gravity of 1.19. Weigh again: the increase in weight is not less than 31.0% of the weight of test specimen.
Silica Gel, Binder-Free
—Silica gel for chromatographic use formulated without a binder, since only activated forms of the silica gel are used as the binding agent.
[NOTE—A suitable grade is available commercially as “Silica Gel H”.]
Silica Gel, Chromatographic
—Use a suitable grade.
[NOTE—A suitable grade is available commercially as “Silica Gel G”.]
Silica Gel
–
Impregnated Glass Microfiber Sheet—Use a suitable grade.
[NOTE—A suitable grade is available commercially as “Seprachrom” Chamber with Type SG ITLC, Product No. 51923, from Gelman Instrument Co., Ann Arbor, MI 48106.]
Silica Gel Mixture, Chromatographic
—A mixture of silica gel with a suitable fluorescing substance.
[NOTE—A suitable grade is available commercially as “Silica Gel GF 254”.]
Silica Gel Mixture, Chromatographic, with Chemically Bound Amino Groups
—Use a suitable grade.
Silica Gel Mixture, Dimethylsilanized, Chromatographic
—Use a suitable grade.
[NOTE—A suitable grade is available as “Silica Gel 60 silanized RP-2 F
254,” from EMD Chemicals,
www.emdchemicals.com.
]
Silica Gel Mixture, Octadecylsilanized Chromatographic
—Use a suitable grade.
[NOTE—A suitable grade is available commercially as KC-18F from Whatman Chemical Separation, Inc., 9 Bridewell Place, Clifton, NJ 07014.]
Silica Gel, Octadecylsilanized Chromatographic
—Use a suitable grade.
[NOTE—A suitable grade is available commercially as “Reversed Phase Uniplates” from Analtech, Inc., Newark, DE 19711.]
Silica Gel Mixture, Octylsilanized, Chromatographic
—Use a mixture of RP-8 chromatographic silica gel with a suitable fluorescing substance agent.
Silica Gel, Porous
—Use a grade suitable for high-pressure liquid chromatography.
[NOTE—A suitable grade for reverse phase high-pressure liquid chromatography is available as “LiChrosorb SI60, Reverse Phase”.]
Silica Microspheres
—Use a suitable grade.
[NOTE—A suitable grade, in a controlled-diameter, spherical, porous form, is available commercially as “Zorbax Sil,” from Agilent,
www.agilent.com.
]
Siliceous Earth, Chromatographic
—
For gas chromatography, use a specially prepared grade meeting the following general description: Purified siliceous earth of suitable mesh size that has been acid- and/or base-washed. It may or may not be silanized.
For column partition chromatography, it is essential that the material be free from interfering substances. If such interferences are known or thought to be present, purify the material as follows: Place a pledget of glass wool in the base of a chromatographic column having a diameter of 100 mm or larger, and add
Purified Siliceous Earth (NF monograph) to a height equal to 5 times the diameter of the column. Add a volume of hydrochloric acid equivalent to one-third the volume of siliceous earth, and allow the acid to percolate into the column. Wash the column with methanol, using small volumes at first to rinse the walls of the column, and continue washing with methanol until the last washing is neutral to moistened litmus paper. Extrude the washed column into shallow dishes, heat on a steam bath to remove the excess methanol, and dry at 105

until the material is powdery and free from traces of methanol. Store the dried material in well-closed containers.
[NOTE—A suitable grade is “Chromosorb W-AW”.]
[NOTE—Suitable silanized grades for gas chromatography are “Gas Chrom Q,” and “Chromosorb W (AW- DMCS-treated).]
[NOTE—A suitable grade for column chromatography is acid-washed “Celite 545,” available from Sigma-Aldrich,
www.sigma-aldrich.com.
]
Siliceous Earth, Chromatographic, Silanized
—Place about 450 g of purified siliceous earth in a large, open, glass crystallizing dish in a vacuum desiccator containing 30 mL of a suitable silane, e.g., a mixture of 1 volume of dimethyldichlorosilane and 1 volume of trimethylchlorosilane, or a mixture of 1 volume of methyltrichlorosilane and 2 volumes of dimethyldichlorosilane. Apply vacuum intermittently for several hours, until no liquid silane remains. Float the treated purified siliceous earth on water, and gently agitate to allow any uncoated particles to sink. Skim the silanized material off the surface, wash it on a sintered-glass funnel with warm methanol until the filtrate no longer is acidic, and dry at 110

.
Silicic Acid,

SiO
2·
xH
2O—
(anhydrous) 60.08—White, amorphous powder. Insoluble in water and in acids; soluble in hot solutions of strong alkalies.
Residue on ignition (Reagent test):

not less than 80.0%.
Nonvolatile with hydrofluoric acid—
Heat 500 mg with 1 mL of sulfuric acid and 10 mL of hydrofluoric acid in a platinum crucible to dryness, and ignite to constant weight: the weight of the residue does not exceed 1.0 mg (0.2%).
Chloride (Reagent test)—
One g shows not more than 0.05 mg of Cl (0.005%).
Sulfate (Reagent test)—
Boil 2 g with 20 mL of dilute hydrochloric acid (1 in 40), filter, neutralize the filtrate with
ammonia TS, and dilute with water to 20.0 mL. A 10-mL aliquot of the solution shows not more than 0.1 mg of SO
4 (0.01%).
Heavy metals (Reagent test)—
Boil 2.5 g with 50 mL of dilute hydrochloric acid (1 in 10) for 5 minutes, filter while hot, and evaporate the filtrate on a steam bath to dryness. Take up the residue in 20 mL of dilute hydrochloric acid (1 in 500), digest for 5 minutes, cool, add water to make 100 mL, and filter. To 40 mL of the filtrate add 10 mL of
hydrogen sulfide TS: any color produced is not darker than that produced by adding 10 mL of
hydrogen sulfide TS to a control containing 0.03 mg of Pb (0.003%).
Iron  241
241 —
—
To 20 mL of the filtrate obtained in the test for
Heavy metals add 1 mL of hydrochloric acid, and dilute with water to 47 mL: the solution shows not more than 0.015 mg of Fe (0.003%).
Silicic Acid—Impregnated Glass Microfilament Sheets with Fluorescent Indicator
—Use a suitable grade.
[NOTE—One example of a suitable grade is “ITLC Type SAF” sheets, available from Gelman Instrument Co., 600 South Wagner Rd., Ann Arbor, MI 48106.]
Silicon Carbide,

SiC—
40.10—In small clean chips, suitable for use in promoting ebullition.
Silicone (75 Percent Phenyl, Methyl)
—Use a suitable grade.
NOTE—A suitable grade is available as “OV-25”.
Silicotungstic Acid, n-Hydrate
(
Tungstosilicic Acid),

H
4Si(W
3O
10)
4·
nH
2O—
2878.17 (anhydrous)—Green powder.
Assay—
Dissolve about 1 g, accurately weighed, in 25 mL of dilute hydrochloric acid (1 in 5). Add 50 mL of a solution of 5 g of cinchonine in dilute hydrochloric acid (1 in 2). Warm on a steam bath for about 30 minutes. Cool, filter through a tared crucible, and ignite at 800

to constant weight. The weight of the residue multiplied by 1.047 is equal to the weight of silicotungstic acid dihydrate in the sample taken. Not less than 98% is found.
Silver Diethyldithiocarbamate,

(C
2H
5)
2NCS
2Ag—
256.14—Use ACS reagent grade.
Silver Nitrate,

AgNO
3—
169.87—Use ACS reagent grade.
Change to read:
Silver Oxide,

Ag
2O—
231.74—Brownish-black, heavy

 USP29
USP29 powder. Slowly decomposes on exposure to light. Absorbs carbon dioxide when moist. Practically insoluble in water; freely soluble in dilute nitric acid and in ammonia; insoluble in alcohol. Store in well-closed containers; do not expose to ammonia fumes or easily oxidizable substances.
Assay—
Dissolve about 500 mg, previously dried at 120

for 3 hours and accurately weighed, in a mixture of 20 mL of water and 5 mL of nitric acid. Dilute with 100 mL of water, add 2 mL of
ferric ammonium sulfate TS, and titrate with 0.1 N ammonium thiocyanate VS to a permanent reddish-brown color. Each mL of 0.1 N ammonium thiocyanate is equivalent to 11.59 mg of Ag
2O: not less than 99.7% of Ag
2O is found.
Loss on drying—
Dry it at 120

for 3 hours: it loses not more than 0.25% of its weight.
Nitrate—
To 500 mg add 30 mg of sodium carbonate and 2 mL of
phenoldisulfonic acid TS, mix, and heat on a steam bath for 15 minutes. Cool,
cautiously add 20 mL of water, render alkaline with
ammonia TS, and dilute with water to 30 mL: any color produced by the test solution is not darker than that produced in a control containing 0.01 mg of NO
3 (0.002%).
Substances insoluble in nitric acid—
Dissolve 5 g in a mixture of 5 mL of nitric acid and 10 mL of water, dilute with water to about 65 mL, and filter any undissolved residue on a tared filtering crucible (retain the filtrate for the test for
Substances not precipitated by hydrochloric acid). Wash the crucible with water until the last washing shows no opalescence with 1 drop of hydrochloric acid, and dry at 105

to constant weight: the residue weighs not more than 1 mg (0.02%).
Substances not precipitated by hydrochloric acid—
Dilute the filtrate obtained in the test for Substances insoluble in nitric acid with water to 250 mL, heat to boiling, and add, dropwise, sufficient hydrochloric acid to precipitate all of the silver (about 5 mL), avoiding any great excess. Cool, dilute with water to 300 mL, and allow to stand overnight. Filter, evaporate 200 mL of the filtrate in a suitable tared porcelain dish to dryness, and ignite: the residue weighs not more than 1.7 mg (0.05%).
Alkalinity—
Heat 2 g with 40 mL of water on a steam bath for 15 minutes, cool, and dilute with water to 50 mL. Filter, discarding the first 10 mL of the filtrate. To 25 mL of the subsequent filtrate add 2 drops of phenolphthalein TS, and titrate with 0.02 N hydrochloric acid VS to the disappearance of any pink color: not more than 0.20 mL is required (0.016% as NaOH).
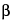 -Sitosterol,
-Sitosterol,
(
22:23 Dihydrostigmasterol),

C
29H
50O—
414.7


[
83-46-5]—White powder. Soluble in chloroform. Store in a freezer.
Specific rotation  781S
781S :
:
between –33

and –39

, determined in a solution containing 0.5 g of test specimen per mL of chloroform.
Soda Lime
—Use Soda Lime (NF monograph).
Sodium,

Na—
At. Wt. 22.98977—Use ACS reagent grade.
Sodium Acetate,

NaC
2H
3O
2·3H
2O—
136.08—Use ACS reagent grade Sodium Acetate Trihydrate.
Sodium Acetate, Anhydrous,

NaC
2H
3O
2—
82.03—Use ACS reagent grade.
Sodium Alizarinsulfonate
(
Alizarin Red S; Alizarin Sodium Monosulfonate),

C
14H
7NaO
7S·H
2O—
360.27—Yellow-brown or orange-yellow powder. Freely soluble in water, with production of a yellow color; sparingly soluble in alcohol.
Sensitiveness—
Add 3 drops of a solution of it (1 in 100) to 100 mL of water, and add 0.25 mL of 0.02 N sodium hydroxide: a red color is produced. Add 0.25 mL of 0.02 N hydrochloric acid: the original yellow color returns.
Sodium Ammonium Phosphate
(
Microcosmic Salt),

NaNH
4HPO
4·4H
2O—
209.07—Colorless crystals or white granules. Freely soluble in water; insoluble in alcohol. Effloresces in air and loses ammonia.
Insoluble matter and ammonium hydroxide precipitate—
Dissolve 10 g in 100 mL of water, add 10 mL of ammonia TS, and heat on a steam bath for 1 hour. If any precipitate is formed, filter, wash well with water, and ignite: the ignited precipitate weighs not more than 1 mg (0.01%).
Chloride (Reagent test)—
One g shows not more than 0.02 mg of Cl (0.002%).
Heavy metals—
Dissolve 3 g in 25 mL of water, add 15 mL of 1 N sulfuric acid, then add 10 mL of
hydrogen sulfide TS: any brown color developed in 1 minute is not darker than that of a control containing 3 mL of
Standard Lead Solution (see
 231
231
) and 0.5 mL of 1 N sulfuric acid (0.001%).
Nitrate—
Dissolve 1 g in 10 mL of water, add 0.1 mL of indigo carmine TS, then add, with stirring, 10 mL of sulfuric acid: the blue color persists for 10 minutes (about 0.005%).
Sulfate (Reagent test, Method II )—
Dissolve 10 g in 100 mL of water, add 5 mL of hydrochloric acid, and filter if necessary: the filtrate yields not more than 5 mg of residue (0.02%).
Sodium Arsenate
(
Arsenic Acid Sodium Salt),

Na
2HAsO
4·7H
2O—
312.01


[
10048-95-0]—Use ACS reagent grade.
Change to read:
Sodium Arsenite,

NaAsO
2—
129.91—White, crystalline

 USP29
USP29 powder. Soluble in water; slightly soluble in alcohol.
Assay—
Transfer about 5.5 g, accurately weighed, to a 500-mL volumetric flask, dissolve in and dilute with water to volume, and mix. Pipet 25 mL of this solution into a suitable container, add 50 mL of water and 5 g of dibasic sodium phosphate, swirl to dissolve, and titrate with 0.1 N iodine VS, adding 3 mL of
starch TS as the endpoint is approached. Each mL of 0.1 N iodine is equivalent to 3.746 mg of As. Between 57.0% and 60.5% is found (equivalent to 98.8% to 104.9% of NaAsO
2).
Chloride (Reagent test)—
One g shows not more than 0.10 mg of Cl (0.01%).
Heavy metals—
Dissolve 200 mg in 8 mL of dilute hydrochloric acid (3 in 8), and evaporate on a steam bath to dryness. Dissolve the residue in 5 mL of dilute hydrochloric acid (2 in 5), and again evaporate to dryness. Dissolve the residue in 10 mL of water, and add 2 mL of diluted acetic acid and 10 mL of
hydrogen sulfide TS. Any brown color produced is not darker than that of a control containing 0.01 mg of added Pb (0.005%).
Iron—
Dissolve 1 g in 20 mL of dilute hydrochloric acid (1 in 5), and add, dropwise, a slight excess of bromine TS. Boil the solution to remove the excess bromine, cool, dilute with water to 40 mL, and add 10 mL of ammonium thiocyanate solution (3 in 10). Any red color produced is not darker than that of a control containing 0.02 mg of added Fe (0.02%).
Sulfide—
Dissolve 1 g in 20 mL of water, and add 5 drops of
lead acetate TS: no brown color is produced (about 0.0005%).
Sulfate (Reagent test, Method II)—
Dissolve 5 g in 100 mL of water, add methyl orange TS, neutralize with 1 N hydrochloric acid, add 3 mL of the acid in excess, and filter: the filtrate yields not more than 3 mg of residue (0.02%).
Sodium Azide,

NaN
3—
65.01—White powder.
Assay—
[Caution—Sodium azide is a potent poison. Its conjugate acid HN3 is more toxic than hydrogen cyanide and is readily liberated from neutral aqueous solutions. Contact of NaH3 or hydrazoic(HN3) with certain metals may produce explosive salts. Work in a well-ventilated hood, and handle the sample with care.
] Dissolve about 100 mg, accurately weighed, in 50 mL of water, and add 3 drops of phenolphthalein. Adjust the pH, if necessary, to 7.0, and add 35.0 mL of 0.1 N perchloric acid. Pipet, while stirring, 2.5 mL of 1.0 M sodium nitrite into the solution, and stir for 15 seconds. Titrate rapidly to the phenolphthalein endpoint with 0.1 N sodium hydroxide. The endpoint should be reached in less than 4 minutes after addition of perchloric acid because HN
3 is readily volatile. Calculate the percentage of azide by the formula:
[(
Np)(
Vp)

(
Ns)(
Vs)](65.01)(100) / 2
C,
where
Np is the normality of perchloric acid solution;
Vp is the volume of perchloric acid, in mL, taken;
Ns is the normality of sodium hydroxide solution;
Vs is the volume, in mL, of sodium hydroxide taken; 65.01 is the molecular weight of sodium azide; and
C is the weight, in mg, of sodium azide. Not less than 98.5% of NaN
3 is found.
Sodium Bicarbonate,

NaHCO
3—
84.01—Use ACS reagent grade.
Sodium Biphenyl,

C
12H
9Na—
176.19—Supplied as a solution (10 percent to 30 percent, w/w) in a mixture of dimethoxyethane and toluene or xylene. The solution is a viscous, dark green liquid.
[NOTE—The solution deteriorates at a rate of about 10% per month. Use only freshly prepared solution.
]
Activity—
Place 20 mL of dry toluene in a titration flask equipped with a magnetic stirring bar and a stopper having a hole through which the delivery tip of a weight buret may be inserted. Add a quantity of sodium biphenyl sufficient to produce a blue color in the mixture, and titrate with amyl alcohol, contained in a weight buret, to the disappearance of the blue color. (Disregard the amounts of sodium biphenyl and amyl alcohol used in this adjustment.) Weigh accurately the weight buret containing the amyl alcohol. Transfer the contents of a vial of well-mixed test specimen to the titration flask, and titrate quickly with the amyl alcohol to the disappearance of the blue color. Weigh the buret to determine the weight of amyl alcohol consumed, and calculate the activity, in mEq per vial, by the formula:
11.25W,
in which
W is the weight of amyl alcohol consumed. Not less than 10% activity is found.
Iodine content—
Add 10 mL to 5 mL of toluene contained in a 125-mL separator fitted with a suitable inert plastic stopcock, and shake vigorously for 2 minutes. Extract gently with three 10-mL portions of dilute phosphoric acid (1 in 3), combining the lower phases in a 125-mL iodine flask. Add
sodium hypochlorite TS, dropwise, to the combined extracts until the solution turns brown, then add 0.5 mL in excess. Shake intermittently for 3 minutes, add 5 mL of freshly prepared, saturated phenol solution, mix, and allow to stand for 1 minute, accurately timed. Add 1 g of potassium iodide, shake for 30 seconds, add 3 mL of starch TS, and titrate with 0.1 N sodium thiosulfate VS: not more than 0.1 mL of 0.1 N sodium thiosulfate is consumed.
Sodium Biphosphate,

NaH
2PO
4·H
2O—
137.99—Use ACS reagent grade Sodium Phosphate, Monobasic.
Sodium Bisulfite
—Use ACS reagent grade Sodium Metabisulfite.
Sodium Bitartrate,

NaHC
4H
4O
6·H
2O—
190.08—White crystals or a crystalline powder. Soluble in cold water.
Assay—
Dissolve about 500 mg, accurately weighed, in 30 mL of water, add phenolphthalein TS, and titrate with 0.1 N sodium hydroxide VS: each mL of 0.1 N sodium hydroxide is equivalent to 19.01 mg of NaHC4H4O6·H2O. Between 99% and 100.5% is found.
Insoluble matter (Reagent test):

not more than 1 mg, from 10 g (0.01%).
Chloride (Reagent test)—
One g shows not more than 0.2 mg of Cl (0.02%).
Heavy metals (Reagent test)—
Dissolve 4 g in 25 mL of water, add 2 drops of phenolphthalein TS, and then add
ammonia TS, dropwise, until the solution is slightly pink. Add 4 mL of 1 N hydrochloric acid, dilute with water to 40 mL, and add 10 mL of
hydrogen sulfide TS: any brown color produced is not darker than that of a control containing 0.04 mg of added Pb (0.001%).
Sulfate (Reagent test, Method I )—
One g shows not more than 0.2 mg of SO4 (0.02%).
Sodium Borate
(Borax; Sodium Tetraborate),

Na
2B
4O
7·10H
2O—
381.37—Use ACS reagent grade.
[NOTE—Certified Borax is available from the National Institute of Standards and Technology, Washington, DC, www.nist.gov, as standard sample No. 187.]
Sodium Borohydride,

NaBH
4—
37.83—White, crystalline solid. Freely soluble in water; soluble (with reaction) in methanol. Its solutions are rapidly decomposed by boiling.
Assay—
POTASSIUM IODATE SOLUTION (0.25 N)—
Dissolve 8.917 g, previously dried at 110

to constant weight and accurately weighed, in water to make 1000.0 mL.
PROCEDURE—
Dissolve about 500 mg, accurately weighed, in 125 mL of sodium hydroxide solution (1 in 25) in a 250-mL volumetric flask, dilute with the sodium hydroxide solution to volume, and mix. Pipet 10 mL of the solution into a 250-mL iodine flask, add 35.0 mL of
Potassium iodate solution, and mix. Add 2 g of potassium iodide, mix, add 10 mL of dilute sulfuric acid (1 in 10), insert the stopper in the flask, and allow to stand in the dark for 3 minutes. Titrate the solution with 0.1 N sodium thiosulfate VS, adding 3 mL of
starch TS as the endpoint is approached. Calculate the amount, in mg, of NaBH
4 in the specimen titrated by the formula:
([(35.0)(0.25)]

0.1
V)4.729,
in which
V is the volume, in mL, of 0.1 N sodium thiosulfate used in the titration. Not less than 98% is found.
Sodium Bromide,

NaBr—
102.89—Use ACS reagent grade.
Sodium Carbonate
—Use Sodium Carbonate, Anhydrous.
Sodium Carbonate, Anhydrous,

Na
2CO
3—
105.99—Use ACS reagent grade.
Sodium Chloride,

NaCl—
58.44—Use ACS reagent grade.
Sodium Chloride Solution, Isotonic
—Use Saline TS.
Change to read:
Sodium Chromate,

Na
2CrO
4·4H
2O—
234.03—Lemon-yellow

 USP29
USP29 crystals. Soluble in water.
Assay—
Accurately weigh about 300 mg, and dissolve in 10 mL of water contained in a 500-mL flask. Add 3 g of potassium iodide and 10 mL of diluted sulfuric acid, and dilute with 350 mL of oxygen-free and carbon dioxide-free water. Titrate the liberated iodine with 0.1 N sodium thiosulfate VS, adding 3 mL of
starch TS as the endpoint is approached. Each mL of 0.1 N sodium thiosulfate consumed is equivalent to 7.802 mg of Na
2CrO
4·4H
2O. Not less than 99% is found.
Insoluble matter (Reagent test):
not more than 1 mg, from 20 g dissolved in 150 mL of water (0.005%).
Aluminum—
Dissolve 20 g in 140 mL of water, filter, and add 5 mL of glacial acetic acid to the filtrate. Add stronger ammonia water until alkaline, and digest for 2 hours on a steam bath. Filter through hardened filter paper, wash thoroughly, ignite, and weigh: the residue weighs not more than 0.8 mg (0.002%).
Calcium—
Determine as directed in the test for calcium for ACS reagent grade Potassium Chromate (0.005%).
Chloride—
Determine as directed in the test for chloride for ACS reagent grade Potassium Chromate (about 0.005%).
Sulfate—
Determine as directed in the test for sulfate for ACS reagent grade Potassium Dichromate, but add 4.5 mL of hydrochloric acid to the water used to dissolve the test specimen: the residue weighs not more than 2.4 mg (0.01%).
Sodium Chromotropate
—See Chromotropic Acid.
Sodium Cobaltinitrite,

Na
3Co(NO
2)
6—
403.94—Use ACS reagent grade.
Sodium Cyanide,

NaCN—
49.01—Use ACS reagent grade.
Sodium 1-Decanesulfonate—
Use a suitable grade.
Sodium Desoxycholate
—Use Bile Salts.
Sodium Dichromate,

Na
2Cr
2O
7·2H
2O (for chromic acid cleaning mixture)—
298.00—Use ACS reagent grade.
Sodium Diethyldithiocarbamate,

(C
2H
5)
2NCS
2Na·3H
2O—
225.31—Use ACS reagent grade.
Sodium Dithionite
—Use Sodium Hydrosulfite.
Sodium Dodecyl Sulfate (Sodium Lauryl Sulfate),

C
12H
25SO
4Na—
288.38—Light yellow, crystalline powder.
Sodium Ferrocyanide,

Na
4Fe(CN)
6·10H
2O—
484.06—Yellow crystals or granules. Freely soluble in water.
Assay—
Dissolve 2 g, accurately weighed, in 400 mL of water, add 10 mL of sulfuric acid, and titrate with 0.1 N potassium permanganate VS. Each mL of 0.1 N potassium permanganate is equivalent to 48.41 mg of Na4Fe(CN)6·10H2O. Not less than 98% is found.
Insoluble matter (Reagent test):

not more than 1 mg, from 10 g (0.01%).
Chloride (Reagent test)—
Dissolve 1 g in 75 mL of water, add a solution prepared by dissolving 1.2 g of cupric sulfate in 25 mL of water, mix, and allow to stand for 15 minutes. To 20 mL of the decanted, clear liquid add 2 mL of nitric acid and 1 mL of
silver nitrate TS: any turbidity produced does not exceed that of a control containing 0.02 mg of Cl, 2 mL of nitric acid, 1 mL of
silver nitrate TS, and sufficient cupric sulfate to match the color of the test solution.
Sulfate—
Dissolve 5 g in 100 mL of water without heating, filter, and to the filtrate add 0.25 mL of glacial acetic acid and 5 mL of
barium chloride TS: no turbidity is produced in 10 minutes (about 0.01% as SO
4).
Sodium Fluorescein

—C
20H
10Na
2O
5—
376.28—Orange-red, hygroscopic powder. Freely soluble in water; slightly soluble in alcohol. Its water solution is yellowish red in color and exhibits a strong yellowish green fluorescence that disappears when the solution is acidified and reappears when the solution is neutralized or made basic.
Loss on drying  731
731 —
—
Dry it at 120

to constant weight: it loses not more than 7.0% of its weight.
Sodium Fluoride,

NaF—
41.99—Use ACS reagent grade.
Change to read:
Sodium Glycocholate,

C
26H
42NNaO
6—
487.60—White to tan

 USP29
USP29 powder. Is hygroscopic. Freely soluble in water and in alcohol.
Specific rotation  781
781 :
:
between +28

and +31

, calculated on the dried basis (it is rendered anhydrous by drying at 100

for 2 hours), determined at 20

in a solution containing 10 mg per mL.
Sodium 1-Heptanesulfonate (1-Heptanesulfonic Acid Sodium Salt)

C
7H
15NaO
3S—
202.25—Use a suitable grade.
Sodium 1-Heptanesulfonate Monohydrate,

C
7H
15NaO
3S·H
2O—
220.26


[
22767-50-6]—Use a suitable grade.
Sodium 1-Hexanesulfonate (1-Hexanesulfonic Acid Sodium Salt),

C
6H
13NaO
3S—
188.22—Use a suitable grade.
Add the following:
 Sodium 1-Hexanesulfonate Monohydrate,
Sodium 1-Hexanesulfonate Monohydrate,

C
6H
13NaO
3S·H
2O—
206.23


[
2832-45-3]—Use a suitable grade.
 USP29
USP29
Sodium Hydrogen Sulfate (Sodium Bisulfate),

NaHSO
4—
120.06


[
7681-38-1]—Freely soluble in water; very soluble in boiling water. It decomposes in alcohol into sodium sulfate and free sulfuric acid. Use a suitable reagent grade.
Sodium Hydrosulfite
(
Sodium Dithionite),

Na
2S
2O
4—
174.11—White or grayish-white crystalline powder. Soluble in water; slightly soluble in alcohol. Gradually oxidizes in air, more readily when in solution, to bisulfite, acquiring an acid reaction. Is affected by light.
Assay—
Accurately weigh about 1 g, dissolve it in a mixture of 10 mL of formaldehyde TS and 10 mL of water contained in a small glass-stoppered flask, and allow to stand for 30 minutes with frequent agitation. Transfer the solution to a 250-mL volumetric flask, add 150 mL of water and 3 drops of
methyl orange TS, and then add, dropwise, 1 N sulfuric acid to a slightly acid reaction. Dilute with water to 250 mL, and mix. To 50.0 mL of the dilution add 2 drops of phenolphthalein TS and just sufficient 0.1 N sodium hydroxide to produce a slight, pink color, then titrate with 0.1 N iodine, adding 3 mL of
starch TS as the indicator. Then discharge the blue color of the solution with 1 drop of 0.1 N sodium thiosulfate, and titrate with 0.1 N sodium hydroxide VS to a pink color: each mL of 0.1 N sodium hydroxide is equivalent to 3.482 mg of Na
2S
2O
4. Not less than 88% is found.
Sulfide—
Add sodium hydroxide solution (1 in 10) to lead acetate TS until the precipitate dissolves. Add 5 drops of this solution to a solution of 1 g of the sodium hydrosulfite in 10 mL of water: no immediate darkening is observed.
Heavy metals—
Dissolve 1 g in 10 mL of water, add 10 mL of hydrochloric acid, and evaporate on a steam bath to dryness. Dissolve the residue in 20 mL of water and 0.5 mL of diluted hydrochloric acid, filter, and add to the filtrate 10 mL of
hydrogen sulfide TS: no darkening is produced. Render the solution alkaline with
ammonia TS: a slight, greenish color may be produced, but not a dark or white precipitate.
Suitability for riboflavin assay—
To each of 2 or more tubes add 10 mL of water and 1.0 mL of a standard riboflavin solution containing 20 µg of riboflavin in each mL, and mix. To each tube add 1.0 mL of glacial acetic acid, mix, add with mixing, 0.5 mL of potassium permanganate solution (1 in 25), and allow to stand for 2 minutes. Then to each tube add, with mixing, 0.5 mL of
hydrogen peroxide TS: the permanganate color is destroyed within 10 seconds. Shake the tubes vigorously until excess oxygen is expelled. If gas bubbles remain on the sides of tubes after foaming has ceased, remove the bubbles by tipping the tubes so that the solution flows slowly from end to end. In a suitable fluorometer, measure the fluorescence of the solution. Then add, with mixing, 8.0 mg of sodium hydrosulfite: the riboflavin is completely reduced in not more than 5 seconds.
Sodium Hydroxide,

NaOH—
40.00—Use ACS reagent grade.
Sodium Hypochlorite Solution
—A solution of sodium hypochlorite (NaOCl) in water. Usually yellow to yellowish-green in color. Has an odor of chlorine. Is affected by light and gradually deteriorates. Store it in light-resistant containers, preferably below 25

.
[Caution—This solution is corrosive and may evolve gases that are corrosive and toxic. It is a powerful oxidant that can react violently with reducing agents. Is irritating and corrosive to skin and mucous membranes.
]
Assay—
Transfer about 3 mL to a tared, glass-stoppered iodine flask, and weigh accurately. Add 50 mL of water, 2 g of potassium iodide, and 10 mL of acetic acid, insert the stopper in the flask, and allow to stand in the dark for 10 minutes. Remove the stopper, rinse the walls of the flask with a few mL of water, and titrate the liberated iodine with 0.1 N sodium thiosulfate VS, adding 3 mL of
starch TS as the endpoint is neared. Each mL of 0.1 N sodium thiosulfate consumed is equivalent to 3.723 mg of NaOCl: not less than 5.25% is found. If it is desired to calculate the percentage of available chlorine, note that each mL of 0.1 N sodium thiosulfate consumed is equivalent to 3.545 mg of available chlorine.
Calcium—
Transfer 10.0 g to a 150-mL beaker, dissolve in 10 mL of water, and add 5 mL of hydrochloric acid and 2 g of potassium iodide. Heat the mixture for 5 minutes, cool, and add 2 mL of 30 percent hydrogen peroxide. Evaporate to dryness, cool, and add 2 mL of hydrochloric acid and 2 mL of 30 percent hydrogen peroxide. Rinse the inner walls of the beaker with a few mL of water, and evaporate to dryness. Take up the residue in 20 mL of water, and filter if necessary. To the filtrate add ammonium hydroxide until the solution is just alkaline, then add 4 drops of ammonium hydroxide and 5 mL of
ammonium oxalate TS: any turbidity produced within 15 minutes does not exceed that in a blank containing 0.1 mg of added Ca carried through the entire procedure (0.001%).
Phosphate (Reagent test)—
Transfer 2 g to a beaker, and add 5 mL of hydrochloric acid and 2 g of potassium iodide. Heat the solution for 5 minutes, and cool. Add 2 mL of 30 percent hydrogen peroxide, and evaporate the solution to dryness. Rinse the walls of the beaker with a few mL of water, and add 2 mL of hydrochloric acid and 2 mL of 30 percent hydrogen peroxide. Evaporate again to dryness: the residue shows not more than 0.01 mg of PO4 (5 ppm).
Sodium Iodate,

NaIO
3—
197.9


[
7681-55-2]—White to yellowish-white powder. Use a suitable reagent grade.
Sodium Lauryl Sulfate—
See Sodium Dodecyl Sulfate.
Sodium Metabisulfite,

Na
2S
2O
5—
190.11—Use ACS reagent grade.
Sodium Metaperiodate,

NaIO
4—
213.89—Use ACS reagent grade Sodium Periodate.
Sodium Methoxide,

CH
3ONa—
54.02—Fine, white powder. Reacts violently with water with evolution of heat. Soluble in alcohol and in methanol.
Assay—
Transfer about 220 mg to a tared, glass-stoppered flask, and weigh accurately. Dissolve the test specimen in about 10 mL of methanol, then add 100 mL of water slowly, with stirring. Add phenolphthalein TS, and titrate with 0.1 N hydrochloric acid VS to a colorless endpoint: each mL of 0.1 N hydrochloric acid VS is equivalent to 5.402 mg of CH3ONa. Not less than 98.0% is found.
Sodium Molybdate,

Na
2MoO
4·2H
2O—
241.95—Use ACS reagent grade.
Sodium Nitrate,

NaNO
3—
84.99—Use ACS reagent grade.
Sodium Nitrite,

NaNO
2—
69.00—Use ACS reagent grade.
Sodium Nitroferricyanide
(
Sodium Nitroprusside),

Na
2Fe (NO)(CN)
5·2H
2O—
297.95—Use ACS reagent grade.
Sodium 1-Octanesulfonate,

C
8H
17NaO
3S—
216.27—Use a suitable grade.
Sodium Oxalate,

Na
2C
2O
4—
134.00—Use ACS reagent grade.
[NOTE—Sodium Oxalate of a quality suitable as a primary standard is available from the Office of Standard Reference Materials, National Institute of Standards and Technology, Washington, DC, www.nist.gov, as standard sample No. 40.]
Sodium
(
tri) Pentacyanoamino Ferrate [Trisodium Aminepentacyanoferrate (3-)],

Na
3[Fe(CN)
5NH
3]—
271.93—Yellow to tan powder. Soluble in water.
Solubility—
Dissolve 500 mg in 50 mL of water, and allow to stand for 1 hour: the solution is clear and free from foreign matter.
Sensitivity—
1,1-DIMETHYLHYDRAZINE STANDARD SOLUTION—Place 500 mL of water in a 1-liter volumetric flask, and add from a buret 1.27 mL of anhydrous 1,1-dimethylhydrazine. Dilute with water to volume, and mix. Pipet 10 mL of this solution into a 100-mL volumetric flask, and dilute with water to volume. Each mL of this solution contains the equivalent of 100 µg of 1,1-dimethylhydrazine.
BUFFER SOLUTION—
Transfer 4.8 g of citric acid monohydrate to a 1-liter volumetric flask, dissolve in water, add 14.6 g of sodium phosphate, swirl to dissolve, and dilute with water to volume.
TEST PREPARATION—
Dissolve 100 mg of sodium (tri)pentacyanoamino ferrate in 100 mL of water.
PROCEDURE—
Into each of five 25-mL volumetric flasks pipet 0, 0.25, 0.50, 1.0, and 1.5 mL, respectively, of 1,1-Dimethylhydrazine standard solution, to each add 15 mL of Buffer solution, and swirl to mix. To each flask, add by pipet 2 mL of Test preparation, mix, dilute with Buffer solution to volume, and allow to stand for 1 hour. Using a suitable spectrophotometer, 1-cm cells, and the solution containing no 1,1-Dimethylhydrazine standard solution as the blank, determine the absorbances of the remaining solutions at 500 nm. Plot the observed absorbance as the ordinate versus the concentration of standard as the abscissa on coordinate paper, and draw the curve of best fit. The plot is linear and the absorbance of the 150-µg solution is not less than 0.65.
Sodium 1-Pentanesulfonate,

C
5H
11NaO
3S·H
2O—
192.21—White, crystalline solid. Soluble in water.
Solubility—
One g, dissolved in 25 mL of water, yields a clear and complete solution.
Sodium Perchlorate,

NaClO
4·H
2O—
140.46—Use ACS reagent grade.
Sodium Peroxide,

Na
2O
2—
77.98—Use ACS reagent grade.
Sodium Phosphate, Dibasic
(
Disodium Phosphate; Disodium Hydrogen Phosphate; Sodium Phosphate,
Dibasic,
Heptahydrate),

Na
2HPO
4·7H
2O—
268.07—Use ACS reagent grade Sodium Phosphate, Dibasic, Heptahydrate.
Sodium Phosphate, Dibasic, Anhydrous
(
Anhydrous Disodium Hydrogen Phosphate) (for buffer solutions),

Na
2HPO
4—
141.96—Use ACS reagent grade Sodium Phosphate, Dibasic, Anhydrous.
Sodium Phosphate, Monobasic
(Sodium Biphosphate; Sodium Dihydrogen Phosphate; Acid Sodium Phosphate; Monosodium Orthophosphate),

NaH
2PO
4·H
2O—
137.99—Use ACS reagent grade.
Sodium Phosphate, Tribasic,

Na
3PO
4·12H
2O—
380.12—Use ACS reagent grade.
Sodium Pyrophosphate,

Na
4P
2O
7·10H
2O—
265.90—Use ACS reagent grade.
Sodium Pyruvate,

CH
3COCO
2Na—
110.04—White to practically white powder or crystalline solid. Soluble in water.
Assay—
Transfer about 300 mg, accurately weighed, to a high-form titration beaker, add 150 mL of glacial acetic acid, and stir until dissolved. Titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically, using a glass electrode and a calomel electrode modified to use 0.1 N tetramethylammonium chloride in methanol as the electrolyte. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 11.00 mg of CH3COCO2Na: not less than 98.0% is found.
Solubility—
Dissolve 1.5 g in 25 mL of water: the solution is clear and complete.
Free acid—
Dissolve 10 g in 150 mL of water, and titrate with 0.5 N sodium hydroxide VS, determining the endpoint potentiometrically: not more than 2.8 mL of 0.5 N sodium hydroxide is consumed (about 1% as C3H4O3).
Sodium Salicylate
—It complies with the specifications under
Sodium Salicylate (USP monograph), and in addition meets the requirements of the following test.
Nitrate—
Dissolve 100 mg in 5 mL of water, and superimpose the solution upon 5 mL of sulfuric acid: no brownish-red color appears at the junction of the two liquids.
Sodium Selenite,

Na
2SeO
3—
172.94—White, odorless, crystalline powder, usually partially hydrated. Freely soluble in water; insoluble in alcohol.
Assay—
Accurately weigh about 180 mg, previously dried at 120

to constant weight, and dissolve it in 50 mL of water in a glass-stoppered flask. Add, successively, 3 g of potassium iodide and then 5 mL of hydrochloric acid, insert the stopper, and allow to stand for 10 minutes. Add 50 mL of water, 50.0 mL of 0.1 N sodium thiosulfate VS, and 3 mL of
starch TS, and immediately titrate with 0.1 N iodine VS to a blue color. Perform a blank determination. The difference in volumes of 0.1 N iodine is equivalent to 4.323 mg of Na
2SeO
3. Between 98% and 101% is found.
Solubility—
One g in 10 mL of water shows not more than a faint haze.
Carbonate—
To 500 mg add 1 mL of water and 2 mL of diluted hydrochloric acid: no effervescence is produced.
Chloride (Reagent test)—
A 500-mg portion shows not more than 0.05 mg of Cl (0.01%).
Nitrate (Reagent test)—
A 200-mg portion dissolved in 3 mL of water shows not more than 0.02 mg of NO3 (0.01%).
Selenate and sulfate (as SO4)—
To 500 mg in a small evaporating dish add 20 mg of sodium carbonate and 10 mL of hydrochloric acid. Slowly evaporate the solution on a steam bath under a hood to dryness. Wash the sides of the dish with 5 mL of hydrochloric acid, and again evaporate to dryness. Dissolve the residue in a mixture of 15 mL of hot water and 1 mL of hydrochloric acid. Proceed as directed under Sulfate in Reagents (Reagent test, Method I), beginning with “Filter the solution.” The test specimen shows no more turbidity than that produced by 0.15 mg of SO4 (0.03%).
Sodium Sulfate
(Glauber's Salt),

Na
2SO
4·10H
2O—
322.20—Use ACS reagent grade.
Sodium Sulfate, Anhydrous,

Na
2SO
4—
142.04—Use ACS reagent grade.
For use in assaying alkaloids by gas-liquid chromatography, it conforms also to the following additional test.
Suitability for alkaloid assays—
Transfer about 10 mg of atropine, accurately weighed, to a 25-mL volumetric flask, dissolve in alcohol, and dilute with alcohol to volume. Pipet 3 mL of the solution into each of two 60-mL separators, and add to each 10 mL of water, 1 mL of 1 N sodium hydroxide, and 10 mL of chloroform. Shake thoroughly, and allow the layers to separate. Filter the organic phase from one separator through phase-separating paper, previously washed with 5 mL of chloroform, supported in a funnel, and collect the filtrate in a suitable container. Add 10 mL of chloroform to the separator, shake thoroughly, and filter the organic layer through the same phase-separating paper, collecting and combining the filtrates in the same container. Designate the combined filtrates as
Solution A. Filter the organic phase from the second separator through 30 g of the Anhydrous Sodium Sulfate, supported on a pledget of glass wool in a small funnel, and previously washed with chloroform, and collect the filtrate in a suitable container. Add 10 mL of chloroform to the separator, shake thoroughly, and filter the organic layer through the same portion of anhydrous sodium sulfate, collecting and combining the two filtrates in the same container. Designate the combined filtrates as
Solution B. Evaporate the two solutions in vacuum to a volume of about 1 mL. Inject an accurately measured volume of
Solution A into a suitable gas chromatography, and record the peak height. Repeat the determination with a second accurately measured volume of
Solution A, record the peak height, and obtain the average of the two results. In a similar manner, determine the peak height of two portions of
Solution B, and obtain the average of the results. The average value obtained for
Solution B is within 5.0% of the value obtained for
Solution A.
Under typical conditions, the gas chromatograph contains a 4-mm × 1.2-m glass column packed with 3% phase G3 on packing S1A. After curing and conditioning, the column is maintained at 210

, the injector port at 225

, and the detector block at 240

during the determinations. The carrier gas is helium, flowing at a rate of 60 mL per minute.
Sodium Sulfate Decahydrate
—Use Sodium Sulfate.
Sodium Tetraphenylborate,

NaB(C
6H
5)
4—
342.22—Use ACS reagent grade.
Sodium Sulfide,

Na
2S·9H
2O—
240.18—Use ACS reagent grade.
Sodium Sulfite
—Use Sodium Sulfite, Anhydrous.
Sodium Sulfite, Anhydrous
(
Exsiccated Sodium Sulfite),

Na
2SO
3—
126.04—Use ACS reagent grade.
Sodium p-Sulfophenylazochromotropate
[
Trisodium Salt of 4,
5-Dihydroxy-3-(
p-sulfophenylazo)
-2,
7-naphthalenedisulfonic Acid],

C
16H
9N
2Na
3O
11S
3·3H
2O—
624.47—Bright red powder. Very soluble in water; insoluble in alcohol. Combines with zirconium oxychloride to form a soluble pink zirconium lake.
[NOTE—The reagent is available as Catalog No. 7309 from Distillation Products Industries, Eastman Organic Chemicals Dept., Rochester, NY 14650. A procedure for its preparation is described in Z. Anal. Chem., 146, 417 (1955).]
Sodium Tartrate,

Na
2C
4H
4O
6·2H
2O—
230.08—Use ACS reagent grade.
Sodium Tetraphenylborate,

NaB(C
6H
5)
4—
342.22—Use ACS reagent grade.
Sodium Tetraphenylboron
—See Sodium Tetraphenylborate.
Sodium Thioglycolate
(
Sodium Thioglycollate),

HSCH
2COONa—
114.10—A white, crystalline powder, having a slight, characteristic odor. Very soluble in water; slightly soluble in alcohol. Is hygroscopic, and oxidizes in air. Store in tight, light-resistant containers. It should not be used if it is pale yellow or darker in color.
Assay—
Accurately weigh about 250 mg, and dissolve in 50 mL of oxygen-free water. Add 5 mL of diluted hydrochloric acid, boil for 2 minutes, cool, and titrate the solution with 0.1 N iodine VS, adding 3 mL of
starch TS toward the end: each mL of 0.1 N iodine is equivalent to 11.41 mg of HSCH
2COONa. Not less than 75% is found.
Insoluble matter—
A solution of 1 g in 10 mL of water is clear, and practically complete.
Sulfide—
Dissolve 500 mg in 10 mL of water in a small flask, add 2 mL of hydrochloric acid, then place a strip of filter paper, moistened with
lead acetate TS, over the mouth of the flask, and bring the solution to a boil: the lead acetate paper is not darkened.
Sodium Thiosulfate,

Na
2S
2O
3·5H
2O—
248.19—Use ACS reagent grade.
Sodium L-Thyroxine
—Use Levothyroxine Sodium (USP monograph).
Sodium 3-(trimethylsilyl)-1-propane sulfonate
(
Sodium 2,
2-dimethyl-2-silapentane-5-sulfonate),

C
6H
15SiNaO
3S—
218.32—Use a suitable grade.
Sodium Tungstate,

Na
2WO
4·2H
2O—
329.85—Use ACS reagent grade.
Soluble Starch
—See Starch, Soluble.
Stannous Chloride,

SnCl
2·2H
2O—
225.65—Use ACS reagent grade.
Starch, Potato
—The starch separated from the tubers of Solanum tuberosum Linné (Fam. Solanaceae). A more or less finely granular powder, consisting of starch grains of characteristic shape and appearance when examined microscopically.
Starch, Soluble
(for iodimetry)—Use ACS reagent grade.
Starch, Soluble, Purified—
White, amorphous powder; under microscopic examination it shows the characteristic form of potato starch. Soluble in hot water; very slightly soluble in alcohol.
TEST SOLUTION FOR DETERMINATION OF PH AND SENSITIVITY—
Stir 2.0 g in 10 mL of water, add boiling water to make 100 mL, and boil for 2 minutes. The hot solution is almost clear. On cooling, the solution may become opalescent or turbid, but does not gel. Use it as the
Test solution.
pH  791
791 —
—
The pH of the
Test solution is between 6.0 and 7.5.
Sensitivity—
Mix 2.5 mL of Test solution, 97.5 mL of water, and 0.50 mL of 0.010 N iodine: a distinct blue color results, and it disappears upon the addition of 0.50 mL of 0.010 N sodium thiosulfate.
Absorbance—
Prepare a pH 5.3 buffer solution by dissolving 43.5 g of sodium acetate (trihydrate) and 4.5 mL of glacial acetic acid in water, transferring the resultant solution to a 250-mL volumetric flask, adding water to volume, and mixing.
Dissolve 1.00 g of Soluble Purified Starch in 2.5 mL of the buffer solution by warming, transfer to a 100-mL volumetric flask, add water to volume, and mix. Add 0.50 mL of this solution to a 100-mL volumetric flask containing about 75 mL of water, 1 mL of 1 N hydrochloric acid, and 1.5 mL of 0.020 N iodine, swirling the flask during the addition. Add water to volume, mix, and allow to stand in the dark for 1 hour. The absorbance of this solution, measured at 575 nm in a 1-cm cell against a blank, is between 0.5 and 0.6.
Reducing substances—
Shake 10.0 g with 100 mL of water for 15 minutes, and allow to settle for about 12 hours. Filter a portion of the supernatant through fine sintered glass. To 50 mL of the filtrate add 50 mL of
alkaline cupric tartrate TS, and boil for 1 to 2 minutes. Filter the resulting cuprous oxide, wash it with hot water and then with alcohol, and dry it at 105

for 2 hours: not more than 47 mg is found, corresponding to 0.7% of reducing sugars as maltose.
Stearic Acid,

C
18H
36O
2—
284.48—Hard, white crystals or amorphous, white powder. Freely soluble in chloroform and in ether; soluble in alcohol and in solvent hexane.
Palmitic acid—
Determine as directed in the Assay under Stearic Acid (NF monograph): not more than 5.0% is found.
Stearyl Alcohol
(
1-Octadecanol),

C
18H
38O—
270.49—White flakes, granules, or crystals. Insoluble in water; soluble in alcohol, in ether, in acetone, and in benzene.
Other requirements—
It conforms to the tests for Acid value, Iodine value, and Hydroxyl value under Stearyl Alcohol (NF monograph).
Strontium Acetate,

Sr(CH
3COO)
2·(1/2)H
2O—
214.72—White, crystalline powder. Soluble in 3 parts of water; slightly soluble in alcohol.
Assay—
Ignite about 3 g, accurately weighed, in a platinum crucible, protecting from sulfur in the flame. Cool, transfer the crucible with the residue to a beaker, and add 50 mL of water and 40.0 mL of 1 N hydrochloric acid VS. Boil gently for 30 minutes or longer, if necessary, filter, wash with hot water until the washings are neutral, add
methyl red TS, and titrate the excess acid with 1 N sodium hydroxide VS. Each mL of 1 N hydrochloric acid is equivalent to 107.4 mg of Sr(CH
3COO)
2·½H
2O: not less than 99% is found.
Insoluble matter (Reagent test):

not more than 2 mg, from 10 g (0.02%).
Free alkali or free acid—
Dissolve 3 g in 30 mL of water, and add 3 drops of phenolphthalein TS: no pink color is produced. Titrate with 0.1 N sodium hydroxide VS to a pink color: not more than 0.30 mL of the 0.1 N sodium hydroxide is required.
Barium—
Dissolve 1 g in 10 mL of water, and add 1 drop of glacial acetic acid and 5 drops of potassium dichromate solution (1 in 10): no turbidity is produced within 2 minutes (about 0.02%).
Calcium—
Ignite 1 g until completely carbonized. Warm the residue with a mixture of 3 mL of nitric acid and 10 mL of water, filter, wash with 5 mL of water, and evaporate the filtrate on a steam bath to dryness. Powder the residue, and dry it at 120

for 3 hours. Reflux the dried powder with 15 mL of dehydrated alcohol for 10 minutes, cool in ice, and filter. Repeat the extraction with 10 mL of dehydrated alcohol. Evaporate the combined filtrates to dryness, add 0.5 mL of sulfuric acid, and ignite: the weight of the residue is not more than 10 mg (0.3% of Ca).
Chloride (Reagent test)—
One g shows not more than 0.1 mg of Cl (0.01%).
Heavy metals (Reagent test):

0.001%.
Iron  241
241 —
—
Dissolve 1.0 g in 45 mL of water, and add 2 mL of hydrochloric acid: the solution shows not more than 0.01 mg of Fe (0.001%).
Alkali salts—
Dissolve 2 g in 80 mL of water, heat to boiling, add an excess of
ammonium carbonate TS, boil for 5 minutes, dilute with water to 100 mL, and filter. Evaporate 50 mL of the filtrate, and ignite: the residue after correcting for the ignition residue from half the volume of the clear ammonium carbonate TS used above, is not more than 3 mg (0.3%).
Nitrate—
Dissolve 1 g in 10 mL of water, add 0.10 mL of indigo carmine TS, and then add 10 mL of sulfuric acid: the blue color persists for 5 minutes (about 0.01% of NO3).
Strontium Hydroxide
(
Strontium Hydroxide Octahydrate),

Sr(OH)
2·8H
2O—
265.76—White, crystalline, free-flowing powder. Sparingly soluble in water. May absorb carbon dioxide from the air. Keep tightly closed.
Assay and carbonate—
Accurately weigh about 5 g, dissolve in 200 mL of warm carbon dioxide-free water in a glass-stoppered, 500-mL flask, add
phenolphthalein TS, and titrate with 1 N hydrochloric acid VS to determine the hydroxide alkalinity. Then add
methyl orange TS, and titrate with 1 N hydrochloric acid VS. Each mL of 1 N hydrochloric acid required to reach the phenolphthalein endpoint is equivalent to 132.9 mg of Sr(OH)
2·8H
2O, and each additional mL of 1 N hydrochloric acid VS required to reach the methyl orange endpoint is equivalent to 73.8 mg of SrCO
3. Not less than 95.0% of Sr(OH)
2·8H
2O and not more than 3.0% of SrCO
3 are found.
Chloride (Reagent test)—
Dissolve 1.0 g in 100 mL of water, and filter if necessary: 1.0 mL of the solution shows not more than 0.01 mg of Cl (0.1%).
Calcium (Reagent test)—
Dissolve 5.0 g in water, and dilute with water to 100 mL, to obtain the
test solution.
 SAMPLE SOLUTION—
SAMPLE SOLUTION—
Dilute 10.0 mL of
test solution with water to 100 mL.
 CONTROL SOLUTION—
CONTROL SOLUTION—
To 10.0 mL of
test solution add 0.50 mg of calcium ion (Ca), and dilute with water to 100 mL.
 PROCEDURE—
PROCEDURE—
Determine the background emission at 416.7 nm: the limit is 0.1%.
Iron—
Dissolve 1 g in warm water, and dilute with water to 100 mL. To 20 mL of this solution add 2 mL of hydrochloric acid and 0.1 mL of 0.1 N potassium permanganate, allow to stand for 5 minutes, and add 3 mL of ammonium thiocyanate solution (3 in 10). Any red color produced is not darker than that of a control containing 0.03 mg of added Fe (0.015%).
For the following test, prepare a test solution as follows. Dissolve 2.0 g in 14 mL of dilute hydrochloric acid (1 in 6), and evaporate on a steam bath to dryness. Take up the residue in 25 mL of water, filter, and dilute with water to 100 mL.
Heavy metals—
To 5.0 mL of
test solution add 0.02 mg of lead (Pb), and dilute with water to 30 mL, to provide the standard. For the test specimen, use 30 mL of
test solution. Adjust each solution with diluted acetic acid or ammonia TS to a pH between 3.0 and 4.0 (using short-range pH paper), dilute with water to 40 mL, and add 10 mL of freshly prepared
hydrogen sulfide TS: any brown color developed in the sample solution is not darker than that in the control solution (0.004%).
Strychnine Sulfate,

(C
21H
22N
2O
2)
2·H
2SO
4·5H
2O—
856.98—Colorless or white crystals, or a white, crystalline powder. Its solutions are levorotatory. One g dissolves in about 35 parts of water, in 85 mL of alcohol, and in about 220 mL of chloroform. Insoluble in ether.
Solubility—
A solution of 500 mg in 25 mL of water is complete, clear, and colorless.
Residue on ignition (Reagent test):

not more than 0.1%.
Brucine—
To 100 mg add 1 mL of dilute nitric acid (1 in 2): a yellow color may be observed, but not a red or reddish-brown color.
Styrene-Divinylbenzene Copolymer Beads
—Neutral, porous, cross-linked beads, 200–400 mesh, molecular weight operating range up to 2,000 (based on beads fully swollen in benzene). Suitable for use in the gel permeation separation of lipophilic polymers and other solutes requiring organic eluant.
[NOTE—A suitable grade is available commercially as “BioBeads S-X” from Bio-Rad,
www.bio-rad.com.
]
Succinic Acid,

C
4H
6O
4—
118.09


[
771-50-6]—Use ACS reagent grade.
Sudan III,

C
22H
16N
4O—
352.39—Red to red-brown powder. Use a suitable grade.
Assay—
When tested by thin-layer chromatography (see
Chromatography  621
621
) with the use of plates coated with chromatographic silica gel mixture and a developing system consisting of a mixture of hexane and ethyl acetate (80:20), and examined under short-wavelength UV light, a single spot is exhibited, with trace impurities.
Sudan IV,

C
24H
20N
4O—
380.44—Brown to reddish-brown powder.
Assay—
Transfer about 25 mg, accurately weighed, to a 100-mL volumetric flask. Dissolve in chloroform, dilute with chloroform to volume, and mix. Dilute 2.0 mL of the resulting solution with chloroform to 50.0 mL. Determine the absorbance of this solution in 1-cm cells at the wavelength of maximum absorbance at about 520 nm, with a suitable spectrophotometer, using chloroform as the blank. Calculate the percentage of Sudan IV in the test specimen taken by the formula:
(100A) / (85C),
in which
A is the absorbance at 520 nm and
C is the concentration of the test specimen in g per L. Not less than 90% is found.
Sulfamic Acid,

HSO
3NH
2—
97.09—Use ACS reagent grade.
Sulfanilic Acid,
 p
p-NH
2C
6H
4SO
3H·H
2O—
191.21—Use ACS reagent grade.
Sulfatase Enzyme Preparation
—Use a suitable grade.
[NOTE—A suitable grade is available commercially under catalog number S-9626 from Sigma-Aldrich, Web site:
www.sigma-aldrich.com.
]
Sulfathiazole Sodium
(
4-Amino-N-2-thiazolylbenzenesulfonamide Sodium Salt),

C
9H
8N
3NaO
2S
2—
277.29


[
144-74-1]—Use a suitable grade.
Sulfosalicylic Acid,

C
6H
3(COOH)(OH)(SO
3H)-1,2,5·2H
2O—
254.22—Use ACS reagent grade.
Sulfur
—Use Precipitated Sulfur (USP monograph).
Sulfur Dioxide Detector Tube—
A fuse-sealed glass tube so designed that gas may be passed through it and containing suitable absorbing filters and support media for an iodine-starch indicator.
Measuring range:
1 to 25 ppm.
Sulfuric Acid,

H
2SO
4—
98.08—Use ACS reagent grade.
Sulfuric Acid, Diluted
(10 percent)—Cautiously add 57 mL of sulfuric acid to about 100 mL of water, cool to room temperature, and dilute with water to 1000 mL.
Sulfuric Acid, Fluorometric
—Use ACS reagent grade Sulfuric Acid that conforms to the following additional test:
Fluorescence—
Using a suitable fluorometer having a sharp cut-off 360-nm excitation filter and a sharp cut-off 415-nm excitation filter, determine the fluorescence of the sulfuric acid in a cuvette previously rinsed with water followed by several portions of the acid under examination: the fluorescence does not exceed that of quinine sulfate solution (1 in 1,600,000,000), similarly measured.
Sulfuric Acid, Fuming,

H
2SO
4 plus free SO
3 having a nominal content of 15%, 20%, or 30% of free SO
3—Use ACS reagent grade (containing between 15.0% and 18.0%, between 20.0% and 23.0%, or between 30.0% and 33.0% of free SO
3).
Sulfurous Acid,

H
2SO
3—
82.08—A water solution of sulfur dioxide—Use ACS reagent grade.
Supports for Gas Chromatography
—See supports for gas chromatography in the
Chromatographic Reagents section under
Chromatography  621
621
.