INDICATORS
Indicators are required in the Pharmacopeial tests and assays either to indicate the completion of a chemical reaction in volumetric analysis or to indicate the hydrogen-ion concentration (pH) of solutions. The necessary solutions of indicators are listed among the Test Solutions, abbreviated “TS.”
Solutions of indicators of the basic type and of the phthaleins are prepared by dissolving in alcohol. With indicators containing an acidic group, the acid must first be neutralized with sodium hydroxide as follows:
Triturate 100 mg of the indicator in a smooth-surfaced mortar with the volume of 0.05 N sodium hydroxide specified in the directions for preparing its Test Solution, or with the equivalent of 0.02 N sodium hydroxide. When the indicator has dissolved, dilute the solution with carbon dioxide-free water to 200 mL (0.05%). Store the solutions in suitably resistant containers, protected from light.
Listed in ascending order of the lower limit of their range, useful pH indicators are: thymol blue, pH 1.2–2.8; methyl yellow, pH 2.9–4.0; bromophenol blue, pH 3.0–4.6; bromocresol green, pH 4.0–5.4; methyl red, pH 4.2–6.2; bromocresol purple, pH 5.2–6.8; bromothymol blue, pH 6.0–7.6; phenol red, pH 6.8–8.2; thymol blue, pH 8.0–9.2; and thymolphthalein, pH 8.6–10.0.
 Alphazurine 2G
Alphazurine 2G
—Use a suitable grade.
 Azo Violet
Azo Violet
[
4-(
p-Nitrophenylazo)
resorcinol], C
12H
9N
3O
4—
259.22—Red powder. It melts at about 193

, with decomposition.
 Bismuth Sulfite
Bismuth Sulfite
—Use a suitable grade.
 Brilliant Yellow
Brilliant Yellow
(
C.I. 24890), C
26H
18N
4Na
2O
8S—
592.49—Orange to rust-colored powder. Soluble in water.
 Bromocresol Blue
Bromocresol Blue
—Use
Bromocresol Green.
 Bromocresol Green
Bromocresol Green
(
Bromocresol Blue; Tetrabromo-m-cresol-sulfonphthalein), C
21H
14Br
4O
5S—
698.01—White or pale buff-colored powder. Slightly soluble in water; soluble in alcohol and in solutions of alkali hydroxides. Transition interval: from pH 4.0 to 5.4. Color change: from yellow to blue.
 Bromocresol Green Sodium Salt
Bromocresol Green Sodium Salt
—Use a suitable grade.
 Bromocresol Purple
Bromocresol Purple
(
Dibromo-o-cresolsulfonphthalein), C
21H
16Br
2O
5S—
540.22—White to pink, crystalline powder. Insoluble in water; soluble in alcohol and in solutions of alkali hydroxides. Transition interval: from pH 5.2 to 6.8. Color change: from yellow to purple.
 Bromocresol Purple Sodium Salt,
Bromocresol Purple Sodium Salt,
C
21H
15Br
2O
5SNa—
562.20—Black powder. Soluble in water. Transition interval: from pH 5.0 to 6.8. Color change: from greenish yellow to purple-violet.
 Bromophenol Blue
Bromophenol Blue
(
3¢,3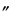 ,5¢,5
,5¢,5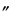 -Tetrabromophenolsulfonphthalein
-Tetrabromophenolsulfonphthalein), C
19H
10Br
4O
5S—
669.96—Pinkish crystals. Insoluble in water; soluble in alcohol and in solutions of alkali hydroxides. Transition interval: from pH 3.0 to 4.6. Color change: from yellow to blue.
 Bromophenol Blue Sodium
Bromophenol Blue Sodium
—The sodium salt of 3
¢,3
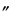
,5
¢,5
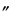
(
Tetrabromophenolsulfonphthalein), C
19H
9Br
4O
5SNa—
646.36—Pinkish crystals. Soluble in water and in alcohol. Transition interval: from pH 3.0 to 4.6. Color change: from yellow to blue.
 Bromothymol Blue
Bromothymol Blue
(
3¢,3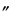 -Dibromothymolsulfonphthalein
-Dibromothymolsulfonphthalein), C
27H
28Br
2O
5S—
624.38—Cream-colored powder. Insoluble in water; soluble in alcohol and in solutions of alkali hydroxides. Transition interval: from pH 6.0 to 7.6. Color change: from yellow to blue.
 Congo Red
Congo Red
—See
Congo Red in the section
Reagents.
 Cresol Red
Cresol Red
(
o-Cresolsulfonphthalein), C
21H
18O
5S—
382.43—Red-brown powder. Slightly soluble in water; soluble in alcohol and in dilute solutions of alkali hydroxides. Transition interval: from pH 7.2 to 8.8. Color change: from yellow to red.
 Crystal Violet
Crystal Violet
(
Hexamethyl-p-rosaniline Chloride), C
25H
30ClN
3—
407.98—Dark-green crystals. Slightly soluble in water; sparingly soluble in alcohol and in glacial acetic acid. Its solutions are deep violet in color.
 Sensitiveness
Sensitiveness
—Dissolve 100 mg in 100 mL of glacial acetic acid, and mix. Pipet 1 mL of the solution into a 100-mL volumetric flask, and dilute with glacial acetic acid to volume: the solution is violet-blue in color and does not show a reddish tint. Pipet 20 mL of the diluted solution into a beaker, and titrate with 0.1 N perchloric acid VS, adding the perchloric acid slowly from a microburet: not more than 0.10 mL of 0.1 N perchloric acid is required to produce an emerald-green color.
 Eosin Y
Eosin Y
(
Indicator grade Eosin Y,
Sodium tetrabromofluorescein), C
20H
6Br
4Na
2O
5—
691.86 [
17372-87-1]—Red to brownish-red pieces or powder. One g dissolves in about 2 mL of water and in 50 mL of alcohol. Dye content about 80%.
 Eriochrome Black T
Eriochrome Black T
[
Sodium 1-(
1-Hydroxy-2-naphthylazo)
5-nitro-2-naphthol-4-sulfonate], C
20H
12N
3NaO
7S—
461.38—Brownish-black powder having a faint, metallic sheen. Soluble in alcohol, in methanol, and in hot water.
 Sensitiveness
Sensitiveness
—To 10 mL of a 1 in 200,000 solution in a mixture of equal parts of methanol and water add sodium hydroxide solution (1 in 100) until the pH is 10: the solution is pure blue in color and free from cloudiness. Add 0.01 mg of magnesium ion (Mg): the color of the solution changes to red-violet, and with the continued addition of magnesium ion it becomes wine-red.
 Eriochrome Black T Trituration
Eriochrome Black T Trituration
—Grind 200 mg of eriochrome black T to a fine powder with 20 g of potassium chloride.
 Litmus
Litmus
—Blue powder, cubes, or pieces. Partly soluble in water and in alcohol. Transition interval: from approximately pH 4.5 to 8. Color change: from red to blue. Litmus is unsuitable for determining the pH of alkaloids, carbonates, and bicarbonates.
 Malachite Green Oxalate,
Malachite Green Oxalate,
[C
23H
25N
2+]
2 · [C
2HO
4
]
2 · C
2H
2O
4—
927.00—The oxalate salt, crystallized with oxalic acid, of a triphenylmethane dye. Dark-green powder, having a metallic luster. Sparingly soluble in water; soluble in glacial acetic acid. Transition interval: from pH 0.0 to 2.0. Color change: from yellow to green.
 Methyl Orange
Methyl Orange
(
Helianthin or
Tropaeolin D), C
14H
14N
3NaO
3S—
327.33—The sodium salt of dimethylaminoazobenzene sulfonic acid or dimethylaminoazobenzene sodium sulfonate. An orange-yellow powder or crystalline scales. Slightly soluble in cold water; readily soluble in hot water; insoluble in alcohol. Transition interval: from pH 3.2 to 4.4. Color change: from pink to yellow.
 Methyl Red (2-
Methyl Red (2-
[[
4-(
Dimethylamino)
phenyl]
azo]
benzoic Acid Hydrochloride), 2-[4-(CH
3)
2NC
6H
4N:N]C
6H
4COOH·HCl—
305.76—Dark-red powder or violet crystals. Sparingly soluble in water; soluble in alcohol. Transition interval: from pH 4.2 to 6.2. Color change: from red to yellow.
 Methyl Red Sodium
Methyl Red Sodium
—The sodium salt of 2-[[4-(dimethylamino)phenyl]azo]benzoic acid. 2-[4-(CH
3)
2NC
6H
4N:N]C
6H
4COONa—
291.28—Orange-brown powder. Freely soluble in cold water and in alcohol. Transition interval: from pH 4.2 to 6.2. Color change: from red to yellow.
 Methyl Yellow
Methyl Yellow
(
p-Dimethylaminoazobenzene), C
14H
15N
3—
225.29—Yellow crystals, melting between 114

and 117

. Insoluble in water; soluble in alcohol, in benzene, in chloroform, in ether, in dilute mineral acids, and in oils. Transition interval: from pH 2.9 to 4.0. Color change: from red to yellow.
 p-Naphtholbenzein
p-Naphtholbenzein
(
4-[

-(
4-Hydroxy-1-naphthyl)
benzylidene]
-1(
4H)
-naphthalenone), (4-HOC
10H
6)C(:C
10H
6-4:O)(C
6H
5)—
374.43—Reddish brown powder. Insoluble in water; soluble in alcohol, in benzene, in ether, and in glacial acetic acid. Transition interval: from pH 8.8 to 10.0. Color change: from orange to green.
 Neutral Red
Neutral Red
(
3-Amino-7-dimethylamino-2-methylphenazine Monohydrochloride), C
15H
16N
4·HCl—
288.78—Reddish to olive-green, coarse powder. Sparingly soluble in water and in alcohol. Transition interval: from pH 6.8 to 8.0. Color change: from red to orange.
 Nile Blue Hydrochloride (Nile Blue A
Nile Blue Hydrochloride (Nile Blue A
,
as the hydrochloride; 5-Amino-9-(
diethylamino)
benzo[
a]
phenoxazin-7-ium chloride), C
20H
20ClN
3O—
353.85—Slightly soluble in alcohol and in glacial acetic acid. Transition interval: from pH 9.0 to 13.0. Color change: from blue to pink.
 Oracet Blue B
Oracet Blue B
(Solvent Blue 19)—A mixture of 1-methyl- amino-4-anilinoanthraquinone (C
21H
16N
2O
2) and 1-amino-4-anilinoanthraquinine (C
20H
14N
2O
2). Where used for titration in non-aqueous media, it changes from blue (basic) through purple (neutral) to pink (acidic).
 Phenol Red
Phenol Red
[
4,
4¢-(
3H-2,
1-Benzoxathiol-3-ylidene)
diphenol,
S,
S-Dioxide], C
19H
14O
5S—
354.38—Crystalline powder, varying in color from bright to dark red. Very slightly soluble in water; freely soluble in solutions of alkali carbonates and hydroxides; slightly soluble in alcohol. Transition interval: from pH 6.8 to 8.2. Color change:from yellow to red.
 Phenolphthalein
Phenolphthalein
[
3,
3-Bis(
p-hydroxyphenyl)
phthalide], C
20H
14O
4—
318.32—White or faintly yellowish-white, crystalline powder. Insoluble in water; soluble in alcohol. Transition interval: from pH 8.0 to 10.0. Color change: from colorless to red.
 Quinaldine Red
Quinaldine Red
(
5-Dimethylamino-2-styrylethylquinolinium Iodide), C
21H
23IN
2—
430.33—Dark blue-black powder. Sparingly soluble in water; freely soluble in alcohol. Melts at about 260

, with decomposition. Transition interval: from pH 1.4 to 3.2. Color change: from colorless to red.
 2-(4-Sulfophenylazo)-1,8-dihydroxy-3,6-naphthalenedisulfonic Acid, Trisodium Salt
2-(4-Sulfophenylazo)-1,8-dihydroxy-3,6-naphthalenedisulfonic Acid, Trisodium Salt
(
4,
5-Dihydroxy-3-(
p-sulfophenylazo)
-2,
7-naphthalenedisulfonic Acid,
Trisodium Salt), C
16H
9N
2O
11S
3Na
3—
570.42—Red powder. Soluble in water.
 Thymol Blue
Thymol Blue
(
Thymolsulfonphthalein), C
27H
30O
5S—
466.59—Dark-colored, crystalline powder. Slightly soluble in water; soluble in alcohol and in dilute alkali solutions.
Acid—Transition interval: from pH 1.2 to 2.8. Color change: from red to yellow.
Alkaline—Transition interval: from pH 8.0 to 9.2. Color change: from yellow to blue.
 Thymolphthalein,
Thymolphthalein,
C
28H
30O
4—
430.54—White to slightly yellow, crystalline powder. Insoluble in water; soluble in alcohol and in solutions of alkali hydroxides. Transition interval: from pH 9.3 to 10.5. Color change: from colorless to blue.
 Xylenol Orange,
Xylenol Orange,
(
N,
N¢-[
3H-2,
1-Benzoxathiol-3-ylidenebis-[(
6-hydroxy-5-methyl-3,
1-phenylene)
methylene]]
bis[
N-(
carboxymethyl)
glycine]
S,
S-dioxide), C
31H
28N
2Na
4O
13S—
760.58—Orange powder. Soluble in alcohol and in water. In acid solution, it is lemon-yellow in color, and its metal complexes are intensely red. It yields a distinct endpoint where a metal such as bismuth, cadmium, lanthanum, lead, mercury, scandium, thorium, or zinc is titrated with edetate disodium.