Assay —
Internal standard solution—
Transfer about 600 mg of hexadecyl hexadecanoate, accurately weighed, to a 200-mL volumetric flask, dissolve in a diluting solution containing 2 volumes of pyridine and 1 volume of propionic anhydride, dilute with the diluting solution to volume, and mix.
Standard preparations—
[NOTE—Use low-actinic glassware.
] Transfer 12-, 25-, 37-, and 50-mg portions of
USP Alpha Tocopherol RS, accurately weighed, to separate 50-mL conical flasks having 19/38 standard-taper ground-glass necks. Pipet 25 mL of the
Internal standard solution into each flask, mix, and reflux for 10 minutes under water-cooled condensers.
Assay preparation—
[NOTE—Use low-actinic glassware.] Transfer about 60 mg of Tocopherols Excipient, accurately weighed, to a 50-mL conical flask similar to the flasks used in preparing the Standard preparations, add 10.0 mL of Internal standard solution, mix, and reflux for 10 minutes under a water-cooled condenser.
Chromatographic system
(see
Chromatography  621
621
)—The gas chromatograph is equipped with a flame-ionization detector and contains a 4-mm × 2-m borosilicate glass column packed with 2% to 5% liquid phase G2 on 80- to 100-mesh support S1AB utilizing either a glass-lined sample introduction system or on-column injection. The column is maintained isothermally at a temperature between 245

and 265

, and the injection port and detector block temperatures are maintained at about 10

higher than the column temperature. The flow rate of dry carrier gas is adjusted to obtain a hexadecyl hexadecanoate peak 30 to 32 minutes after sample introduction.
[NOTE—Cure and condition the column as necessary.
]
System suitability—
Chromatograph a sufficient number of injections of the Assay preparation, as directed under Calibration, to ensure that the resolution, R, between the major peaks occurring at retention times of approximately 0.50 (delta tocopheryl propionate) and 0.63 (beta plus gamma tocopheryl propionates), relative to hexadecyl hexadecanoate at 1.00, is not less than 2.5.
Calibration—
Chromatograph 2- to 5-µL portions of each
Standard preparation, and record the peak areas as directed for
Procedure. Calculate the relative response factor,
F , for each concentration of the
Standard preparation taken by the formula:
( A S / A D )( C D / C S ),
in which
C D and
C S are the concentrations, in mg per mL, of hexadecyl hexadecanoate and of
USP Alpha Tocopherol RS, respectively, in the
Standard preparation. Successively chromatograph a sufficient number of portions of each
Standard preparation to ensure that the factor,
F , is constant within a range of 2.0%. Prepare a relative response factor curve by plotting
F versus the alpha tocopheryl propionate peak area.
Procedure—
Inject a suitable portion (2 µL to 5 µL) of the
Assay preparation into the chromatograph, and record the chromatogram. Measure the areas under the 4 major peaks occurring at relative retention times of approximately 0.50, 0.63, 0.76, and 1.00, and record the values as
a 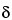
,
a 
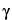
,
a 
, and
a D, corresponding to delta tocopheryl propionate, beta plus gamma tocopheryl propionates, alpha tocopheryl propionate, and hexadecyl hexadecanoate, respectively. Calculate the quantity, in mg, of each tocopherol form in the Tocopherols Excipient taken by the formulas:
delta tocopherol = (10
C D /
F )(
a 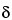
/
a D );
beta plus gamma tocopherols = (10
C D /
F )(
a 
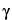
/
a D );
alpha tocopherol = (10
C D /
F )(
a 
/
a D ),
in which
F is obtained from the relative response factor curve (see
Calibration) for each of the corresponding areas under the delta, beta plus gamma, and alpha tocopheryl propionate peaks produced by the
Assay preparation.
[NOTE—The relative response factor for delta tocopheryl propionate and for beta plus gamma tocopheryl propionates has been determined empirically to be the same as for alpha tocopheryl propionate.
]