2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-, monohydrochloride, [4
S-(4

,4a

,5

,5a

,6
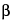
,12a

)]-.
4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride



[
2058-46-0].