Identification—
Dissolve 5 mg in 5 mL of water, add 2 mL of tribasic sodium phosphate solution (1 in 200), mix, then add 10 drops of
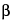
-naphthoquinone-4-sodium sulfonate solution (1 in 40): a blackish brown color is produced.
Assay—
Ferric chloride solution—
Dissolve 6.7 g of ferric chloride in dilute hydrochloric acid (1 in 100) in a 100-mL volumetric flask. Add dilute hydrochloric acid (1 in 100) to volume, mix, and filter.
Standard preparation—
Dissolve a suitable quantity of
USP Deferoxamine Mesylate RS, accurately weighed, in water to obtain a solution having a known concentration of about 1000 µg per mL.
Assay preparation—
Dissolve about 50 mg of Deferoxamine Mesylate, accurately weighed, in water to make 50.0 mL, and mix.
Procedure—
Pipet 2 mL each of the
Standard preparation, the
Assay preparation, and water to provide a blank, into separate 25-mL volumetric flasks. To each flask add 3 mL of
Ferric chloride solution, dilute with water to volume, and mix. Concomitantly determine the absorbances of the solutions from the
Standard preparation and the
Assay preparation against the blank, in 1-cm cells, at the wavelength of maximum absorbance at about 485 nm, with a suitable spectrophotometer. Calculate the quantity, in mg, of C
25H
48N
6O
8·CH
4O
3S in the portion of Deferoxamine Mesylate taken by the formula:
0.05C(AU / AS),
in which
C is the concentration, in µg per mL, of
USP Deferoxamine Mesylate RS in the
Standard preparation, and
AU and
AS are the absorbances of the solutions from the
Assay preparation and
Standard preparation, respectively.