Identification—
A: Thin-Layer Chromatographic Identification Test  201
201 —
—
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture.
Test solution—
Dissolve about 150 mg of Extract in 10 mL of chloroform. Apply 10 µL to the plate.
Standard solution 1—
Prepare a solution of USP Pygeum Extract RS in chloroform having a concentration of about 15 mg per mL.
Standard solution 2—
Prepare a solution of
USP 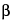 -Sitosterol RS
-Sitosterol RS in chloroform having a concentration of about 2 mg per mL.
Developing solvent system:
methylene chloride in a saturated chamber.
Spray reagent—
Prepare a solution of sulfuric acid and water (1:1).
Procedure—
Develop the chromatogram to a length of not less than 15 cm, and dry the plate in a current of air. Spray the plate with
Spray reagent, and heat the plate at 100

for 10 minutes. Examine the plate under white light: the chromatogram obtained with the
Test solution shows one red-violet zone turning to grayish-brown near the origin that corresponds in color and
RF value to that in the chromatogram of
Standard solution 1, and one red-violet zone turning to grayish-brown at an
RF of about 0.08 corresponding in color and
RF value to that in the chromatogram of
Standard solution 2; above these spots a grayish-brown zone may be present, corresponding in color and
RF value to that in the chromatogram of
Standard solution 1; and other colored zones of varying intensities may be observed in the chromatogram of the
Test solution.
B:
The retention time of the peak for docosyl ferulate in the chromatogram of the Test solution corresponds to that in the chromatogram of the Standard solution, as obtained in the Content of docosyl ferulate.
Content of sterols—
Derivatizing solution:
a mixture of bis(trimethylsilyl)acetamide and trimethylchlorosilane (9:1).
Internal standard solution—
Prepare a solution containing 2 mg per mL of 5

-cholestane in chloroform.
System suitability solution—
Prepare a solution containing about 2 mg per mL each of campesterol, stigmasterol, and
USP 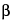 -Sistosterol RS.
-Sistosterol RS. Transfer 2.0 mL of this solution and 2.0 mL of
Internal standard solution to a 10-mL volumetric flask, and dilute with chloroform to volume. Evaporate about 500 µL of this solution to dryness using a stream of nitrogen. Dissolve the residue in 80 µL of
Derivatizing solution and 20 µL of pyridine. Allow to stand for not less than 10 minutes at room temperature.
Standard stock solution—
Prepare a solution of
USP 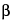 -Sitosterol RS
-Sitosterol RS in chloroform having a known concentration of about 2.0 mg per mL.
Standard solution—
Transfer 2.0 mL of the Standard stock solution and 2.0 mL of the Internal standard solution to a 10-mL volumetric flask, and dilute with chloroform to volume. Evaporate about 500 µL of this solution to dryness using a stream of nitrogen. Dissolve the residue in 80 µL of Derivatizing solution and 20 µL of pyridine. Allow to stand for not less than 10 minutes at room temperature.
Test solution—
Transfer an accurately weighed quantity of about 100 mg of Extract into a 100-mL, round-bottomed flask. Add 2.0 mL of
Internal standard solution and 20 mL of diluted hydrochloric acid. Attach a condenser, and reflux in a bath at 100

for 30 minutes. Cool the solution to room temperature, and adjust by the addition of about 5 mL of 10 N sodium hydroxide to a pH of 8. Extract twice using 50 mL of ether each time, wash the collected organic phases with 50 mL of water, and evaporate the organic phase to dryness under vacuum. Dissolve the residue with 4 mL of chloroform, and transfer to a cartridge containing 500 mg of packing L8 that has been conditioned with a 2-column volume of
n-hexane.
[NOTE—A suitable cartridge is Chromabond NH2, manufactured by Macheray Nagel, or equivalent.
] Collect the eluate. Elute twice with a 1-column volume of a mixture of chloroform and isopropanol (2:1). Combine the eluates, and evaporate to dryness. Dissolve the residue in 10 mL of chloroform. Evaporate about 500 µL of this solution to dryness under a stream of nitrogen. Dissolve the residue with 80 µL of
Derivatizing solution and 20 µL of pyridine. Allow to stand for not less than 10 minutes at room temperature.
Chromatographic system (see Chromatography  621
621 )—
)—
The gas chromatograph is equipped with a split injection system, a flame-ionization detector, and a 0.32-mm × 30-m capillary column coated with a G27 phase of 0.25-µm thickness. The chromatograph is programmed as follows. Initially the temperature of the column is equilibrated at 250

for 5 minutes, then the temperature is increased at a rate of 5

per minute to 320

. The injection port temperature and detector temperature are both maintained at 285

. The carrier gas is helium, with a flow rate adjusted to obtain a retention time of about 19 minutes for
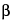
-sitosterol, a split ratio of 1:50, and the makeup gas is helium. Chromatograph the
System suitability solution, and record the peak responses as directed for
Procedure: the relative retention times are about 0.66, 0.94, 0.96, and 1.00 for 5

-cholestane, campesterol, stigmasterol, and
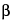
-sitosterol, respectively; the resolution,
R, between campesterol and stigmasterol is not less than 2; the column efficiency is not less than 150,000 theoretical plates for the 5

-cholestane peak; and the tailing factor for each relevant peak is not more than 2.0.
Procedure—
Separately inject equal volumes (about 2 µL) of the
Standard solution and the
Test solution into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Identify the signals corresponding to the relevant analytes by comparison with the chromatograms obtained with the
System suitability solution.
Separately calculate the percentages of campesterol, stigmasterol, and
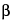
-sitosterol respectively in the portion of Extract taken by the formula:
200C/W(RU / RS),
in which
C is the concentration of
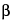
-sitosterol, in mg per mL, in the
Standard solution; W is the weight, in mg, of the Extract taken to prepare the
Test solution; RU is the ratio of the appropriate sterol peak to the internal standard in the chromatogram of the
Test solution; and
RS is the ratio of the
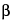
-sitosterol peak to the 5

-cholestane internal standard in the chromatogram of the
Standard solution. Calculate the total content of sterols in percentage by adding the individual percentages.
Content of docosyl ferulate—
Solution A—
Use a mixture of methanol and water (95:5).
Solution B—
Use filtered and degassed acetonitrile.
Mobile phase—
Use a mixture of
Solution A and
Solution B (85:15). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
Standard solution—
Dissolve an accurately weighed quantity of USP Docosyl Ferulate RS in chloroform, and dilute quantitatively, and stepwise if necessary, with acetonitrile to obtain a solution having a known concentration of about 0.02 mg per mL. Filter with a 0.45-µm membrane or finer porosity.
Test solution—
Weigh approximately 250 mg of Extract. Add 5 mL of chloroform, and quantitatively transfer to a 25-mL volumetric flask. Dilute with acetonitrile to volume, and mix. Filter with a 0.45-µm membrane or finer porosity, discarding the first 4 mL of the filtrate.
Chromatographic system (see Chromatography  621
621 )—
)—
The liquid chromatograph is equipped with a 323-nm detector and a 4-mm × 25-cm column that contains packing L7, and is maintained at a temperature of 25

. The flow rate is about 1.0 mL per minute. Chromatograph the
Standard solution, and record the peak responses as directed for
Procedure: the column efficiency for the peak of docosyl ferulate is not less than 1700 theoretical plates; and the tailing factor for docosyl ferulate is not more than 2.0.
Procedure—
Separately inject equal volumes (about 20 µL) of the
Standard solution and the
Test solution into the chromatograph, record the chromatograms, and measure the areas of the analyte peaks. Calculate the percentage of docosyl ferulate in the portion of Extract taken by the formula:
2500C/W(rU / rS),
in which
C is the concentration, in mg per mL, of
USP Docosyl Ferulate RS in the
Standard solution; W is the weight, in mg, of the portion of Extract taken to prepare the
Test solution; and
rU and
rS are the peak responses for docosyl ferulate in the
Test solution and the
Standard solution, respectively.