Procedure—
Proceed as directed in the chapter. Develop the chromatograms in
Developing solvent system until the solvent front has moved not less than 12 cm, and dry the plate in a current of air. Examine the plate under UV light at 254 nm: the chromatogram obtained from the
Test solution shows one main zone corresponding in
RF value to the zone due to dodeca-2
E,4
E,8
Z,10
E-tetraenoic acid isobutylamide and dodeca-
2E,4
E,8
Z,10
Z-tetraenoic acid isobutylamide in the chromatogram of
Standard solution 1 and below this zone there are several other zones due to

,

,
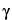
,
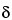
-unsaturated isobutylamides. Spray the plate with
Spray reagent, and then heat the plate at 100

for 5 minutes. Examine the plate under long-wavelength UV light: the zone due to dodeca-2
E,4
E,8
Z,10
E-tetraenoic acid isobutylamide and dodeca-2
E,4
E,8
Z,10
Z-tetraenoic acid isobutylamide turns blue-black, and below this zone there are several other zones due to

,

,
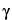
,
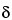
-unsaturated isobutylamides (not detectable in
Echinacea pallida) that turn violet (unlike the corresponding zones in the chromatogram of
Echinacea angustifolia that are mostly yellowish due to

,

-unsaturated isobutylamides). A zone due to

-sitosterol that corresponds in
RF value to the principal spot in the chromatogram of
Standard solution 2 is also observed.